Daniel V. Esposito and many of his colleagues at the US National Institute of Standards and Technology have scrimped and saved on the microscopic photoelectric challenge of producing hydrogen.
Instead of simple electron flow, they have managed to convert light into energy that can be used when the Sun sets. Hydrogen can be used for large scale energy storage and transport. Using a PEC or photo-electro-chemical cell, the photons of light are used to split water into its constituent gases at 12.5% efficiency.
Professor Esposito reckons, "it's been estimated that such a cell would be extremely expensive--thousands of dollars per square meter - and they also had issues with stability." He, on the other hand, has avoided rapid corrosion of the semiconductors and gone for more stability, economy and therefore more efficiency in the system.
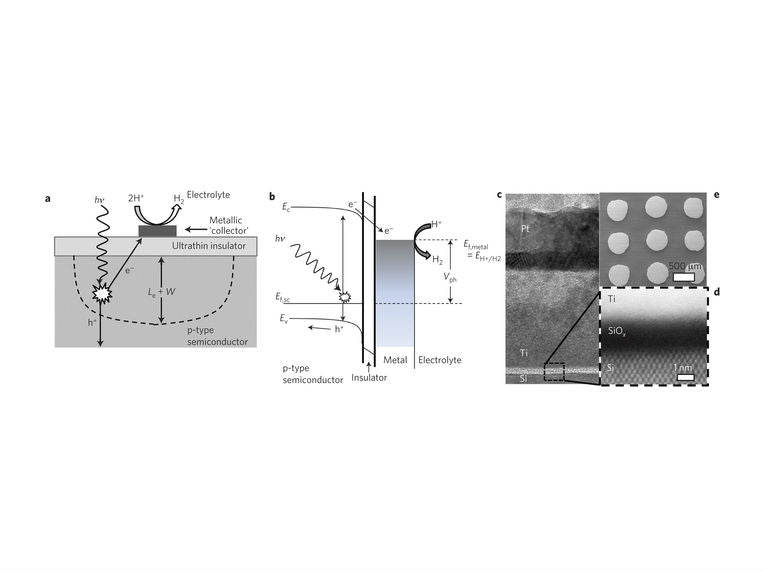
Based on silicon, an MIS or metal-insulator semiconductor is used with a thin, uniform layer of insulating silicon dioxide to collect the photons. The metal is still expensive, with titanium covered with platinum in tiny arrays on top of the insulator. The platinum should last for a long time, as it catalyses the hydrogen production.
The rate of production of molecular hydrogen, H2, was measured across the microscopic array as electrons travelled through the silicon dioxide insulator. This spillover of electrons enhanced the production way beyond any similar "cheap" cell. This taught the NIST team that the 2.9% efficiency was remarkably stable, despite its low level when compared with expensive cells. Highly efficient and cheap water-splitting could be just around the corner, using the techniques invented in this new cell.
The paper can be viewed in the journal, Nature Materials.