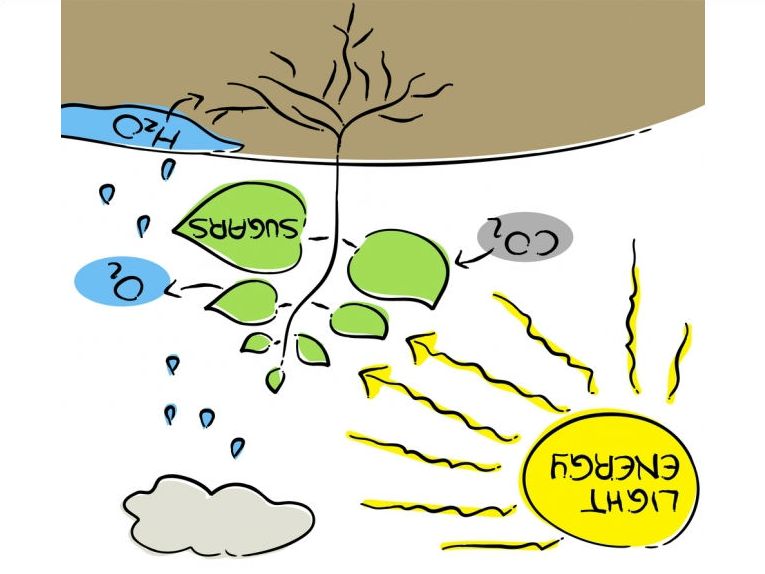
It might seem complicated but reverse photosynthesis is pretty basic. You take the carbohydrates that plants and bacteria normally produce in photosynthesis and use light energy to convert them enzymatically into smaller products. These substances could be the most useful substances that we need in industry such as biofuels and the monomers for plastics production. It does seem that the oil industry could finally be displaced by mere enzymes.
This research has been building for many years at the University of Copenhagen and is fronted now by D. Cannella, K. B. MÃ ¶llers, N.-U. Frigaard, P. E. Jensen, M. J. Bjerrum, K. S. Johansen & C. Felby, from that prestigious institution and the Chalmers University of Technology in GÃ ¶teborg. Professor Felby is the brains behind the whole project, billed forbiddingly in Nature Communications as Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme.
Reverse photosynthesis does not produce carbon dioxide and water as you might expect. Here is an exposition of how photosynthetic processes could solve the energy needs of a future generation! It simply uses the enzymes that would make the reaction work in the direction of polysaccharides and allows the power of light energy to force the reaction backward to produce smaller compounds from even very complex biomass substances such as cellulose, insect chitin, glycogen or starch. Cellulose of course is the basic component of all plant cell walls. So we can use this new
process to break down even waste into small substances such as glucose or alcohol. As an example, methanol or ethanol could be processed simply into fuel or plastic. What a gas!
The name of the widespread powerful enzymes involved is the metalloenzymes, LPMO (lytic polysaccharide monooxygenases), based on a copper atom. They simply oxidise the carbohydrate using electron donors from chlorophyll, helped by some hydrolases. They are natural enzymes, found in the obvious saprophytic candidates- fungi, viruses and bacteria that would normally be slowly breaking down the dead biomass in our ecosystems. That mechanism attracted the Danes, as the natural electron donors were so varied that the enzymes were obviously quite promiscuous
about their choice of donor. Hence they tried the use of light (to excite chlorophyll) just as in plant cells and found El Dorado!
The whole process has been obvious to us, but hidden, it seems, in plain sight.
The skill of this research is in winkling out how the reversing can be accomplished in a relatively efficient way. The efficiency lies in of a 100-fold increase in the enzyme activity which the authors note has never been observed before. Obviously these neglected areas of our biology can gift us immeasurable benefit if he potential of Professor Felby’s research is fulfilled. The suggestion is that this light-induced oxidation process may even be quite natural in our global Carbon Cycle, despite our ignorance of such a process to date.