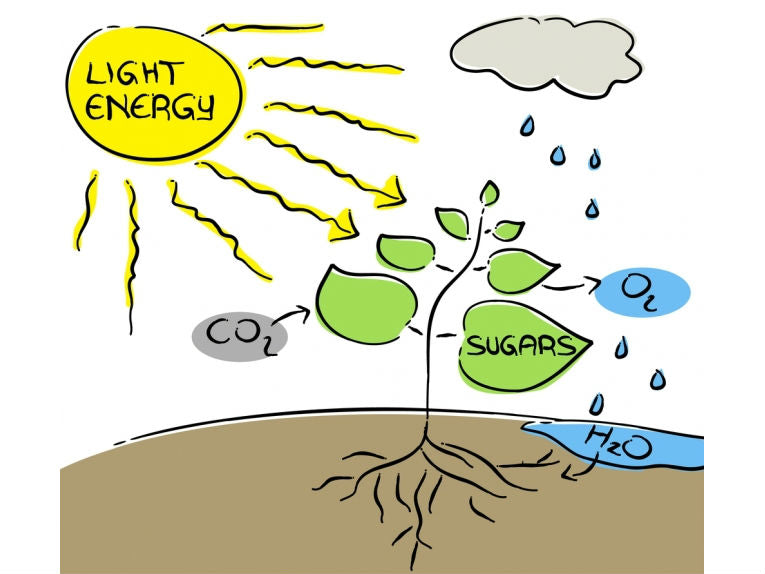
When wind and Sun have been utilised, eco-experts have often asked, why not the air, the rain and the Sun. CO2 and water are used by plants to manufacture all of their energy, using only a few minerals for the basic process. While we learn this at school, scientists have been struggling to learn how to adapt the catalysts to function in an artificial leaf system.
History in this subject can be misleading. The lack of outright success means that possibly the breakthrough, when it comes, will be out of the blue, rather like photosynthesis itself. Nevertheless, there is a chance that the continual modifications have recently managed to produce expensive, but usable hydrogen.
This results when water is broken up in the Hill reaction of photosynthesis. Plants go on to cycle it into carbohydrate production, but we would have to sell it at a profit to pay for the expensive metals probably used in catalysis. The oxygen is less important as a saleable item.
It sometimes seems ridiculous that such a simple move has such extravagant and expensive taste in catalysts. The plants use cheap magnesium while we potter about with indium, molybdenum, nickel, platinum, titanium, iridium or cobalt.

Professor John Turner started the whole ball rolling at Golden, Colorado. He still seems near to the elusive energy-efficient goal of energy from light; Credit: Credit: National Renewable Energy Laboratory, US
The future is a little more exciting and on the edge. Futures tend to be bright but the old experiments could give insight into new problems. We need a storage molecule for a photosynthetic battery and the plants' carbohydrate is totally unsuitable. It must burn cleanly, be much more pure and produce more energy per unit space. The obvious choice is hydrogen itself, but perhaps that is too simple.
Professor John Turner began his work in Golden, Colorado before and during 1998. Using the solar panels from the Mars rovers, with platinum catalysts, he achieved a magnificent conversion rate. A full 12% of the sun's energy was converted into bubbling hydrogen. Green plants can manage precisely 10% of his energy production. The biggest problem was the oxygen. Hydrogen explodes, as the Hindenburg balloon found out.
The equipment only performed for 20 hours, but the acid used would ideally be replaced with water to produce a longer-lasting method. Unfortunately that didn't happen. So now we have a Californian Joint Centre for Artificial Photosynthesis with a $122 million grant, larger than any previous investment. Massachusetts Institute of Technology are only one of the many others who are trying and have actually produced working systems.
Mechanisms to provide this cheapest of fuels vary. Collecting the light so far has been silicon, stacked to increase the poor electron-volt output. Oxygen reacts with it to form insulating "sand," terminating the reactions, so antioxidant adds to the initial small cost. The original expensive apparatus from Turner used gallium arsenide and gallium indium phosphide.
This opportunistically caught wavelengths that plants can only dream of, giving out the large amounts of hydrogen he achieved. However, these catalysts also oxidise, like the silicon, leaving a limited life as one of the key problems with the artificial leaf, still leaving us puzzling today.

When John Turner used his early system, the catalysts used wavelengths of light that plants cannot use and achieved remarkable efficiency. Plants use the UV, red and blue wavelengths, leaving the green reflected as the that inspiring colour we recognise as a photosynthesising organ system (Leaf!) - Credit: Shutterstock
The metal oxides, being oxides, can't be oxidised, so it was suggested that, for cheapness, they would be the next light-gatherers. That dream was typically human .Some salty water and some rusted metals in the sun et voila! No such luck, so far.
The Turner problem was expensive platinum. While plants simply catalyse with enzymes containing iron, Monte Helm in Washington State has revolutionised the catalysis with cheaper nickel. It works "like a dream" but is still being integrated into the photosynthetic apparatus. Meanwhile molybdenum sulphide is employed, just in case the energetic nickel catalyst fails to "live the dream."
Another catalyst is now being employed for oxygen production. Replacing platinum here is iridium oxide, but it's almost equally expensive. Manganese or cobalt oxides would copy a plant-like system, but the plant enzyme is very slow. Turner is now working on the area of these metal oxides in perfect crystals. This makes the job of the electrons easier as they can't be produced efficiently in imperfect systems.
All in all, a realistic photosynthesis still exists only in our green relatives. One of the many teams working produced the ideal. Sun Catalytix invoked a river water system using nickel, molybdenum and zinc catalyst with a cobalt-borate oxygen catalyst that produces 2.5% efficiency and lasts a week. Progress since 1998 has been retrograde as well as forward it seems. The aim is to cut the costs to $3 per kilo for the hydrogen production and increase the efficiency to more like that of Turner's!
The dream of "free energy" is still far away, although MIT. are expecting a system useful for the developing world to be ready soon. Professor Daniel Nocera created his artificial leaf at playing card size. It floats on a pool of water and could power a house's needs for 24 hours. The problem of the cost in regions where income is very low can be overcome for a lucky few. Tata, the Indian conglomerate, have already decided to use the system in a small power plant. Leaf or power station, either way it is the future, as long as we can cost the operation. Meanwhile, back in Colorado, a leaf from titanium and magnesium co-alloyed hematite thin film is exciting John Turner. Perhaps we could make ethanol from the hydrogen and use up our burgeoning CO2 in the process. That would be a popular move, at least.
The apple leaf (below), one of the most useful chemical factories known to the human race. Credit: Shutterstock