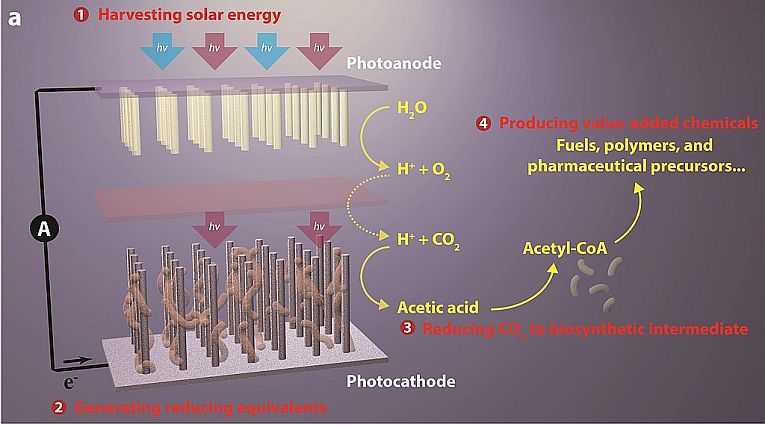
photoanode, followed by a reduction (2), in which the traditional Hill Reaction occurs to absorb carbon dioxide with released hydrogen from water. Then (3), the acetyl co-enzyme A is created, again as in normal photosynthesis and abracadabra, any oil product can be mimicked (4)! Berkeley’s image; Credit: © Nano Lett, 2015 American Chemical Society
Often have we travelled along the leafy paths of photosynthesis, seeking the golden fleece of energy. With hydrogen catalysts and CO2 absorbers, artificial leaves for sticking in ponds or complex substrates for large-scale production, few researchers ever kept the original photosynthesisers. Now a hybrid system is said to be the next big thing in renewables, using the bacterial action of Sporosoma ovata and a nanotechnology of silicon and titanium oxide wire. Just one technique involved could appropriate carbon dioxide emissions and convert them into fuel.
The light-capturing wire absorbs energy for the bacteria, which are then naturally able to use the energy interface
to build carbon dioxide into acetate ions. These products normally convert via acetyl co-enzyme A to various carbohydrates or other bacterial products, depending on species. In this case, Lui Chong and his colleagues from the University of California, Berkeley, US, have persuaded the hybrid system, aerobically and at neutral pH, to be very stable in synthesising n-butanol, a PHB polymer and 3 natural isoprenoids. These alcohols and acids are just the beginning of a huge range of possible organic fuels and polymers that would be capable of cheaply undercutting the oil industry. The important paper can be read as Nanowire–Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals.
Using a hybrid system has transformed the green car market, so we can imagine great progress once this idea takes hold outside of Berkeley with different bacteria and any fuel imaginable. The products of artificial leaves were seen here as the fuel source for hydrogen–powered cars. Hopefully the carbon dioxide production of all such fuels will always be zero, following the last thousand years of bitter experience.
Other closely-related fields of research that may still prove positive were hinted at from our articles on the historyof use of expensive metals in artificial photosynthesis, from platinum and iridium to titanium and magnesium!